FOR IN VITRO DIAGNOSTIC USE
This instruction for use (IFU) must be read carefully prior to use. Instruction for use must be carefully followed. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions for use.
The test is for Professional Use Only.
PACKING SPECIFICATION
20 Tests/ Kit
INTENDED USE
This kit is used for in vitro qualitative detection of SARS-CoV-2 antigen in human nasopharyngeal swab and oropharyngeal swab samples.
Positive result from the test needs further analyze with clinical history of patient and other diagnostic information to determine patient infection status. Positive value is only a reference guide for clinical diagnosis. The test results only reflect the current state of the sample. Negative result cannot exclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
This test is only for clinical laboratory, medical institutions use or for real-time inspection by professional medical staff, not for home testing. It cannot be used as the basis for the diagnosis and exclusion of pneumonia caused by SARS-CoV-2 and is not suitable for general population screening.
The laboratory testing of SARS-CoV-2 should meet the requirements of the "Laboratory testing for SARS-CoV-2 in suspected human cases" and other requirements, and pay attention to the biosecurity.
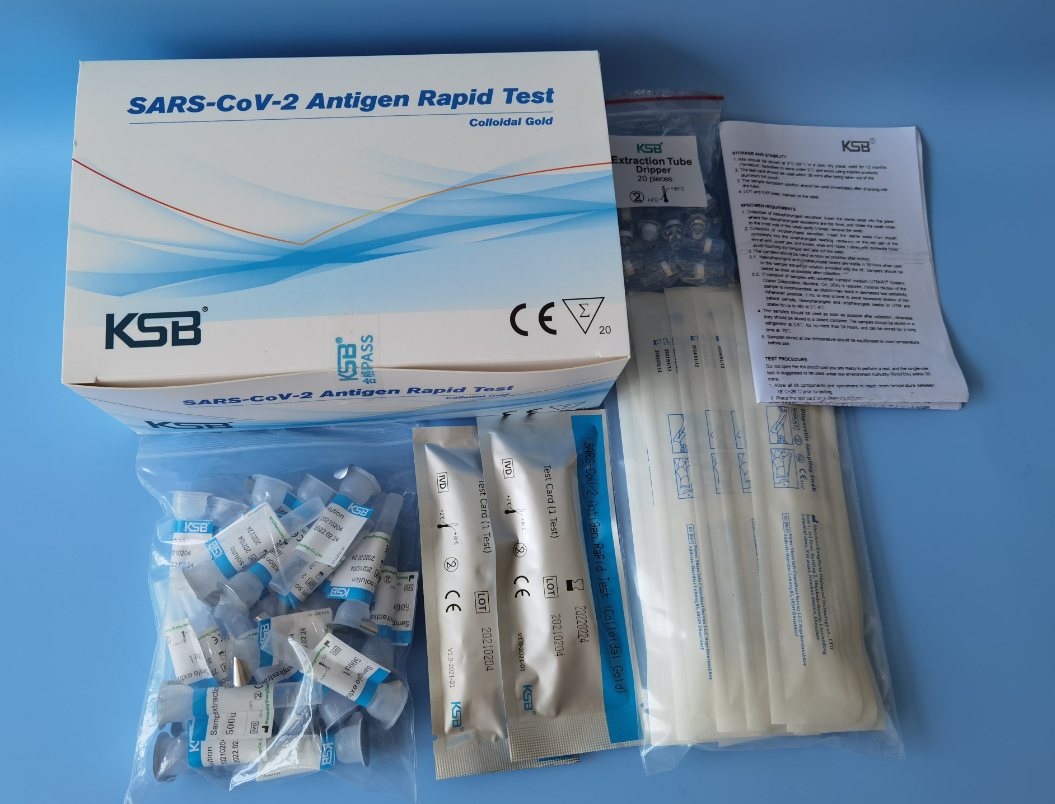
REAGENTS AND MATERIALS SUPPLIED
1. Main components:
Specification Ingredients | 20 tests/kit |
Test card with desiccant in a sealed foil pouch | 20 |
Sample extraction solution | 20 |
Extraction tube holder | 1 |
Extraction Tube | 20 |
Dripper | 20 |
Swab | 20 |
Instruction for use | 1 |